
Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid Derivatives
Lithium Aluminum Hydride (LAH, LiAlH4) For the Reduction of Carboxylic Acid Derivatives
- Lithium aluminum hydride (LiAlH4) is a strong reducing agent similar to, but stronger than, sodium borohydride (NaBH4)
- Like NaBH4, lithium aluminum hydride will reduce aldehydes and ketones to alcohols.
- Unlike NaBH4, it will also reduce carboxylic acids, esters, lactones, acid halides and anhydrides to primary alcohols
- LiAlH4 will also reduce nitriles and amides to amines
- Finally it can open epoxides as well as reduce alkyl halides to alkanes.

Table of Contents
-
- Lithium Aluminum Hydride, LiAlH4
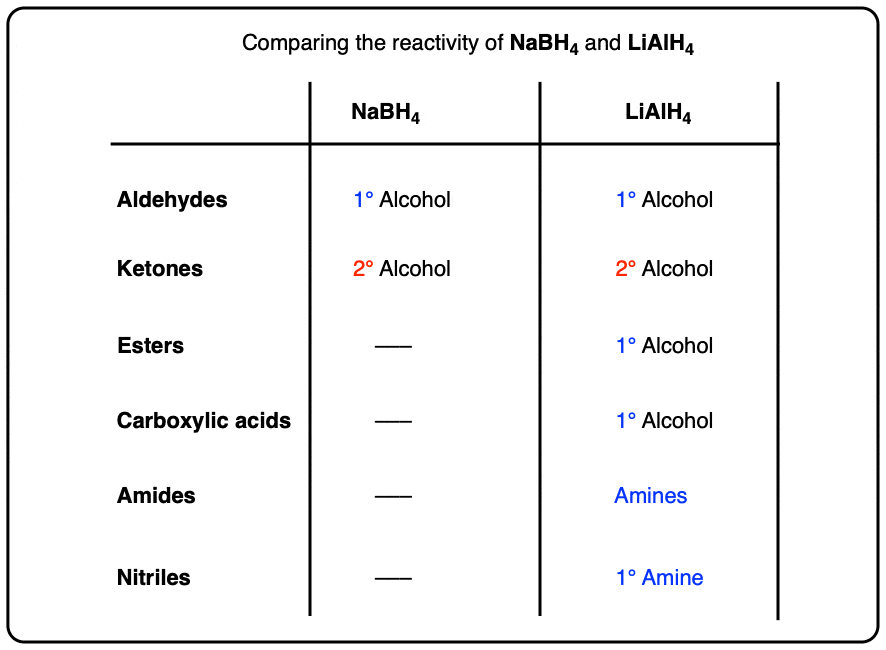
- LiAlH4 vs NaBH4
- Reduction of Carboxylic Acids by LiAlH4 - The Mechanism
- Mechanism for the Reduction of Esters by LiAlH4
- Reduction of Amides to Amines by LiAlH4
- Reduction of Nitriles
- Other Reactions of LiAlH4
- Summary
- Notes
- Quiz Yourself!
- (Advanced) References and Further Reading
1. Lithium Aluminum Hydride, LiAlH4
Lithium aluminum hydride (LiAlH4) is a strong reducing agent with a particular utility for carboxylic acid derivatives.
If you’re not familiar with what is meant by “reducing agent” in organic chemistry, a refresher can be found here. (See article - Oxidation and Reduction in Organic Chemistry. Short version - a reducing agent forms C-H bonds while breaking C-O bonds)
What do you think the full Lewis structure of LiAlH4 looks like?
Of all reagents, LiAlH4 most resembles sodium borohydride. [See article - Sodium borohydride (NaBH4 ) for the reduction of aldehydes and ketones] Hopefully this should not come as a huge shock, since aluminum is right below boron on the periodic table.
A key property of these reagents is that they act as sources of hydride ion H(-) in their reactions with carbonyls and other electrophiles.
To an untrained eye, that might seem strange, since the negative charge appears to be on aluminum. Why might that be?
This might be a good opportunity to remind ourselves that formal charge is not necessarily the same thing as electron density.
- The electronegativity of hydrogen is 2.2.
- The electronegativity of aluminum is 1.61
So although the aluminum bears a negative formal charge (See article - How To Calculate Formal Charge), it is the hydrogens which bear a partial negative charge (greater electron density) and aluminum bears a partial positive charge (lesser electron density).
Given that both lithium aluminum hydride and sodium borohydride behave like a source of H(-), see if you can draw a proper electron pushing arrow for the reaction of LiAlH4 with the weak acid, water.
Which might you expect to be a more reactive donor of hydride ion - borohyride [BH4-] , or AlH4(-).
(There is a bit more to it than what this answer suggests. The counter-ion is important too. Lithium borohydride (LiBH4) is more reactive than NaBH4. More in this Note 1 below)
2. NaBH4 vs LiAlH4
LiAlH4 will reduce aldehydes and ketones just like NaBH4 .
For practical reasons [Note 2] NaBH4 is much more convenient to use for these reactions and there is no advantage to using LiAlH4 unless you also plan on reducing every other functional group in sight. (As my undergraduate instructor Prof. Walter Szarek was fond of saying, “Using LiAlH4 for this reaction is like using a sledgehammer to kill a fly!”).
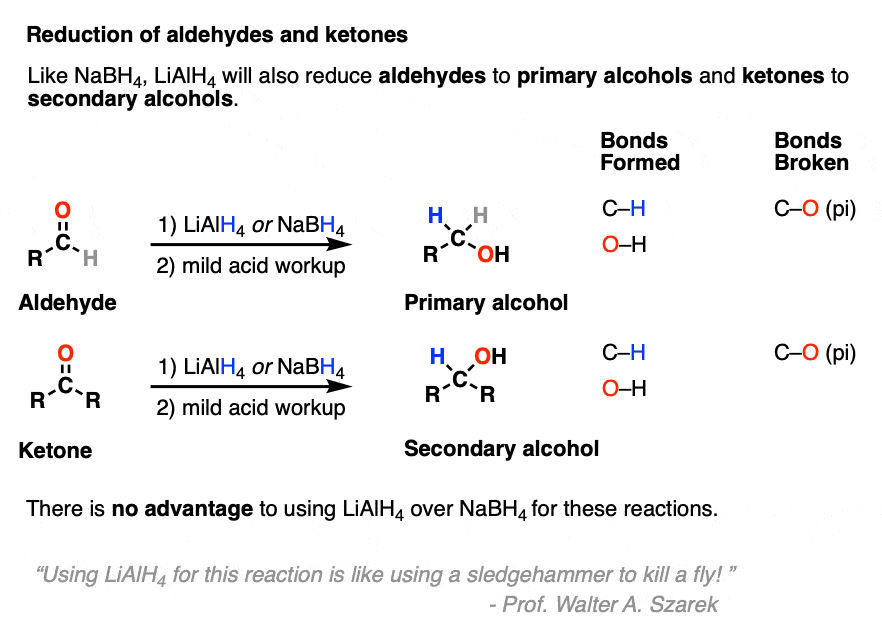
LiAlH4 will also perform reductions that NaBH4 is unable to do, or at the very least, do them much more quickly.
One key difference is in the reduction of esters to primary alcohols, which NaBH4 does only slowly (if at all).
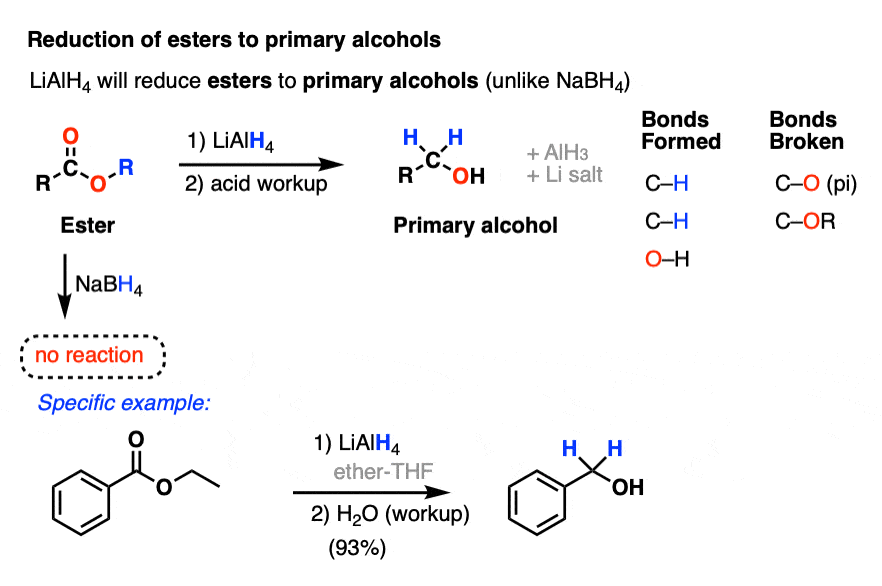
A detailed, reproducible step-by-step procedure from Organic Syntheses can be found here
A second reaction that LiAlH4 will perform that NaBH4 will not is the reduction of carboxylic acids to primary alcohols.
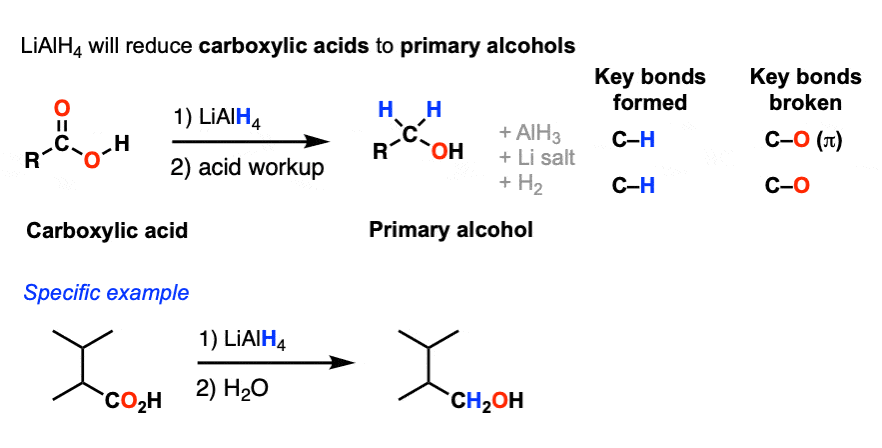
(Reference here)
Other key reactions include
- acid halides to primary alcohols
- anhydrides to primary alcohols
As well as reductions of nitriles, amides, epoxides, and alkyl halides (and more, which we won’t cover)
3. Reduction of Carboxylic Acids By LiAlH4 - The Mechanism
So how does the reaction of LiAlH4 with carboxylic acids work?
Let’s start with the basics. Carboxylic acids are acids. Lithium aluminum hydride is strongly basic.
What might be the very first reaction to happen here?
Yes - an acid-base reaction.
Recall that the pKa of H2, the conjugate acid of hydride (H-) is about 36 whereas the pKa of the carboxylic acid is around 4. Since acid-base reactions are favored when a stronger acid will be converted to a weaker acid, this will rapidly generate the carboxylate salt (the conjugate base of the carboxylic acid) and hydrogen gas. (See article: How to Use a pKa Table).
Acid-base reactions of LiAlH4 tend to be violently exothermic, and generate (flammable) hydrogen gas, besides. For these reasons extreme caution is used when handling LiAlH4 and it is never left out on the bench for any extended period, as it will react with water vapor from the air. Fires can result. [Note 3]. That’s one key reason why NaBH4 is typically used for simple reductions - at cold temperatures, it reacts slowly and controllably with alcoholic solvents, unlike LiAlH4.
In the presence of most nucleophiles, formation of a carboxylate signals the end of the reaction. We’ve seen that carboxylates will not undergo addition with most nucleophiles. (See article - Nucleophilic Acyl Substitution)
LiAlH4 is an exception. [Note 4] In the first step, a hydride from aluminum forms a new C-H bond, breaking the C-O pi bond. In the second step, the C-O pi bond is re-formed, resulting in breakage of the C-O sigma bond.
Nucleophilic acyl substitution on carboxylates is usually extremely difficult due to the strongly basic nature of the O(2-) leaving group, but the strong O-Al bond and aluminum’s strongly Lewis acidic character likely greatly assists here.
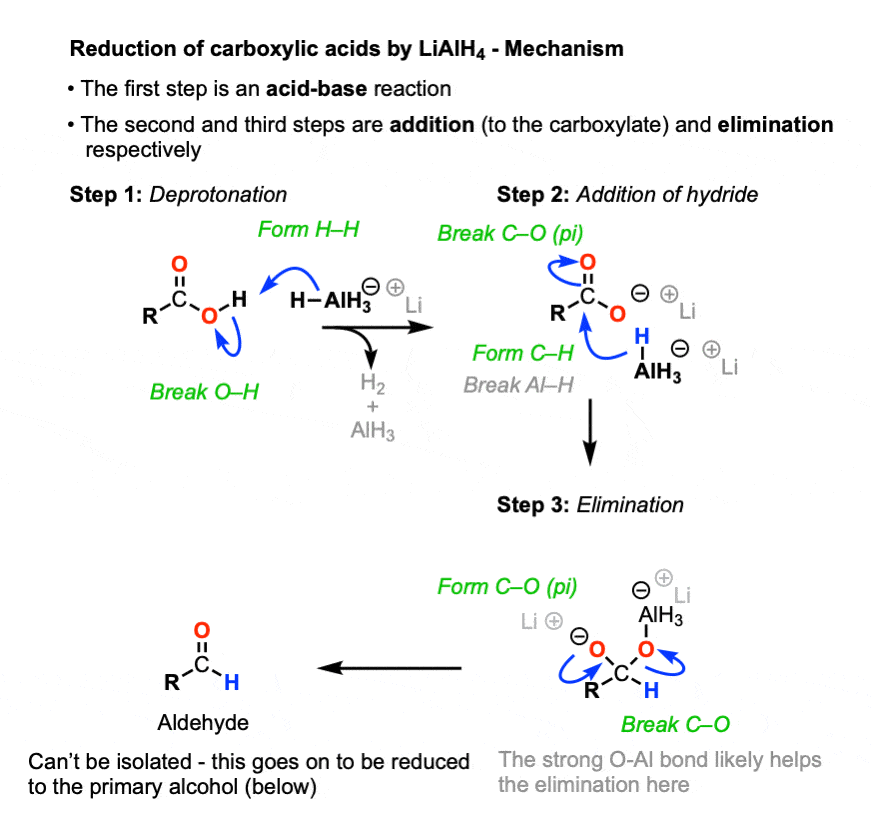
Elimination results in an aldehyde, which quickly undergoes another reduction. In theory, LiAlH4 has four equivalents of hydride that can be transferred, so it wouldn’t be incorrect to draw AlH3 as the source of hydride here. In practice, an excess of LiAlH4 is generally used, and it’s fine to draw this step of the mechanism as occurring from another equivalent of AlH4(-).
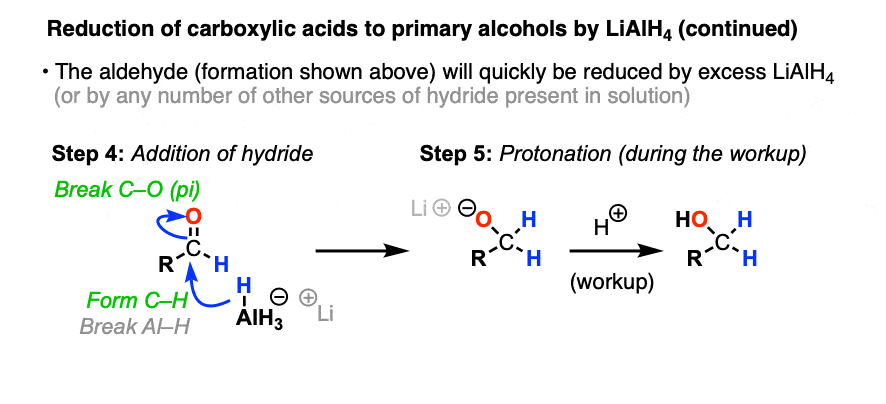
This gives us the conjugate base of a primary alcohol (an alkoxide) coordinated to aluminum.
To get our alcohol back, we perform a quench of the reaction with water, which protonates the alkoxide and gives us our neutral alcohol.
That’s how it works on paper, anyway!
In practice, the workup is a little bit more complicated because aluminum salts make bitching emulsions that make isolation difficult unless they are completely hydrolyzed (yet another reason to just use NaBH4 if you can!) The Fieser workup is the industry standard, but there are others. [Note 3]
4. The Mechanism For the Reduction of Esters by LiAlH4
The mechanism for the reaction of LiAlH4 with esters is even simpler.
Addition of hydride to the ester [form C-H, break C-O(pi)] followed by elimination of alkoxide [form C-O(pi), break C-O] gives the aldehyde.
As we’ve seen, LiAlH4 does not stop there. It has enough equivalents of hydride to eat aldehydes for breakfast, lunch, and dinner and have a little bit left over for dessert.
After the aldehyde has been consumed, a mildly acidic workup gives the primary alcohol.
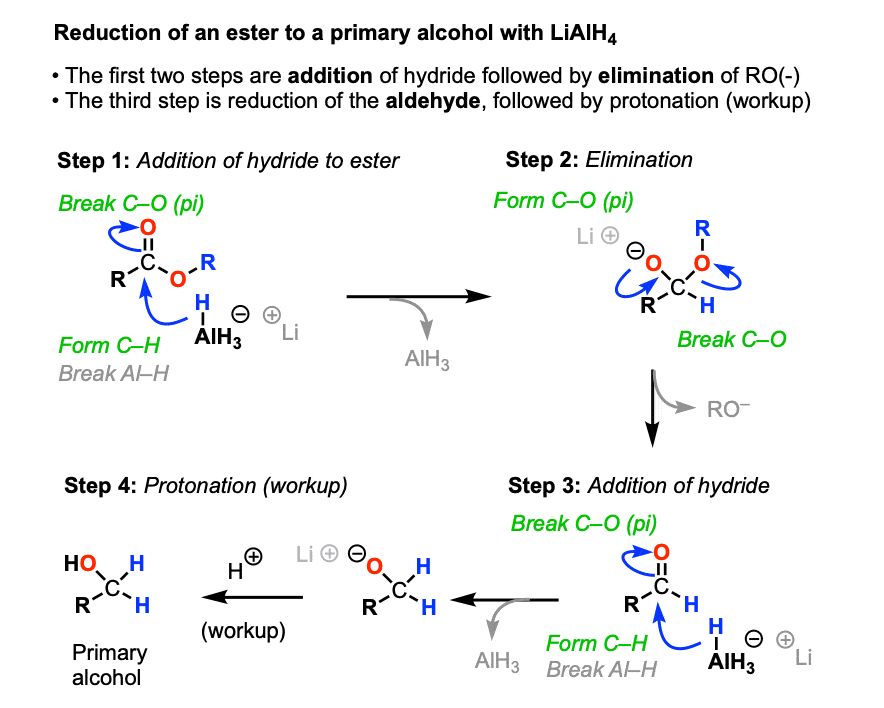
It is possible to get reduction of esters to stop at the aldehyde stage by adding some groups to the aluminum that serve as a “fat suit”. DIBAL (Di-isobutylaluminum hydride, DIBAL-H) is a classic reagent for these purposes. (See article - Di-isobutyl aluminum hydride)
LiAlH4 will also reduce acid halides and anhydrides to primary alcohols through a mechanism similar to that of esters.
It’s also possible to perform the partial reduction of acid halides to aldehydes through using the related reagent LiAlH(Ot-Bu)3 (See article - Lithium tri(t-butoxy)Aluminum Hydride)
5. Reduction of Amides To Amines by LiAlH4
Amides are another class of functional groups that are difficult to reduce.
LiAlH4 will reduce amides to amines, however. NaBH4 won’t touch them.
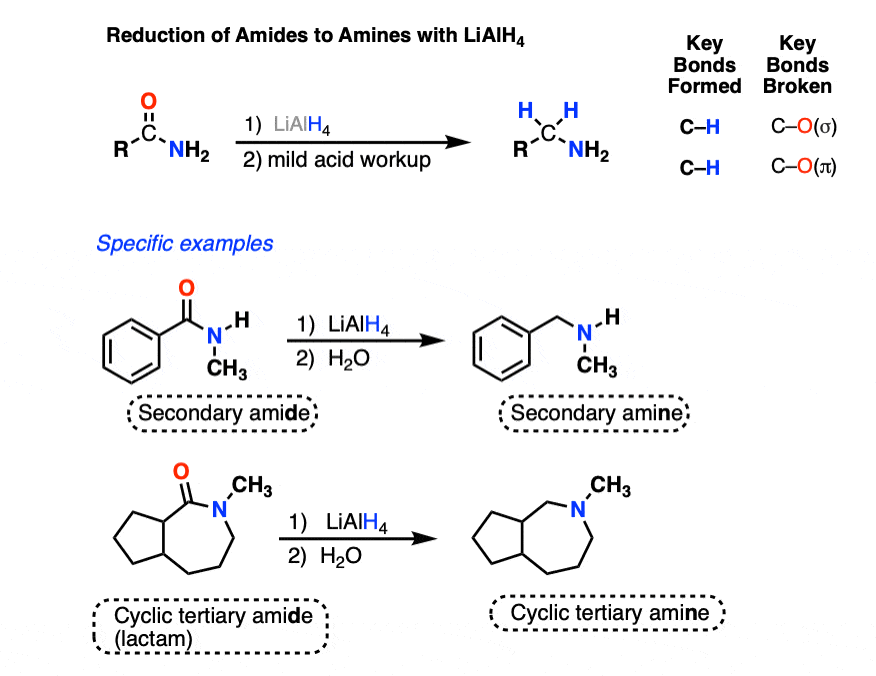
The N-H bonds of primary and secondary amides are weakly acidic (pKa about 17) and will undergo deprotonation by LiAlH4 to give their conjugate bases followed by reduction to the amine (using the mechanism shown below). [To see the deprotonation, hover here and an image will pop up or click the link ]
(Tertiary amides, which lack an acidic hydrogen, will not undergo a preliminary acid-base step.)
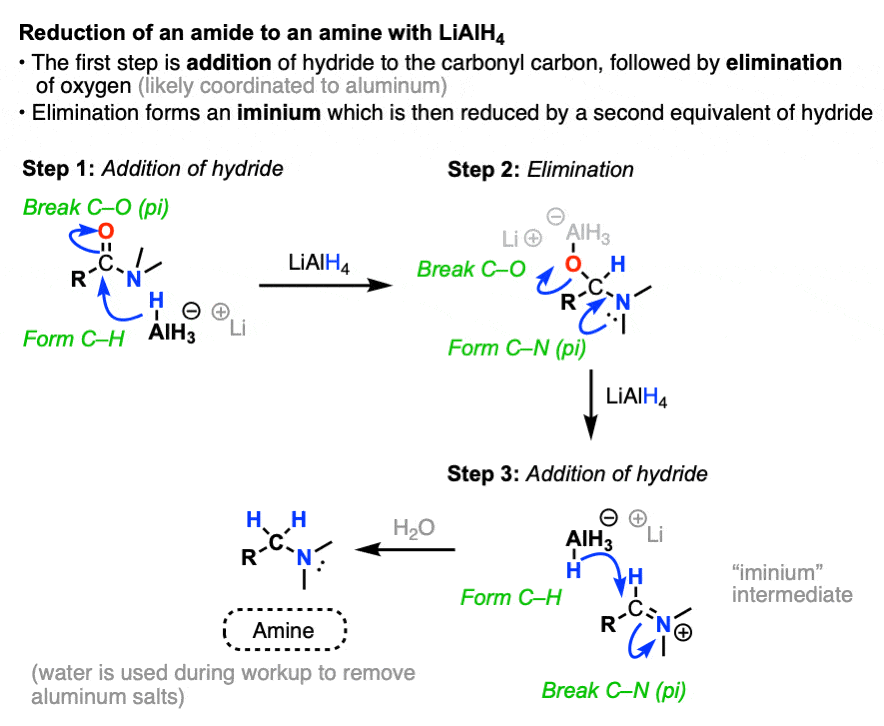
The first step of the reduction of amides is the familiar addition of hydride to carbon (form C-H, break C-O pi) to give a tetrahedral intermediate.
In nucleophilic acyl substitutions, the second step is elimination of the weakest base to give a new pi bond.
This usually occurs via formation of C-O (pi) with loss of a leaving group. So might initially guess that the C-O pi bond is the one to form here, eliminating R2N(-) and giving us an intermediate aldehyde.
But that’s not what happens! Instead, it’s the C-O bond is broken, and a C-N pi bond is formed, giving an iminium intermediate and an alkoxide leaving group. (Note 5).
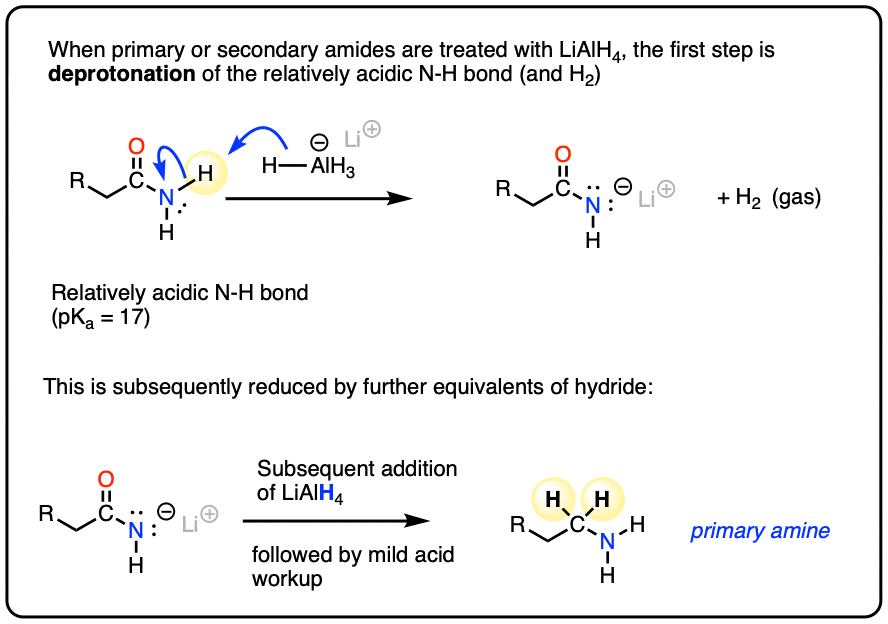
Like aldehydes, the iminium intermediate doesn’t last long in the presence of all those hydrides.
The final product obtained after workup (to remove aluminum salts) is an amine.
The type of amine depends on the amide precursor:
- Primary amides give primary amines
- Secondary amides give secondary amines
- Tertiary amides give tertiary amines
For a detailed Organic Syntheses procedure, click here.
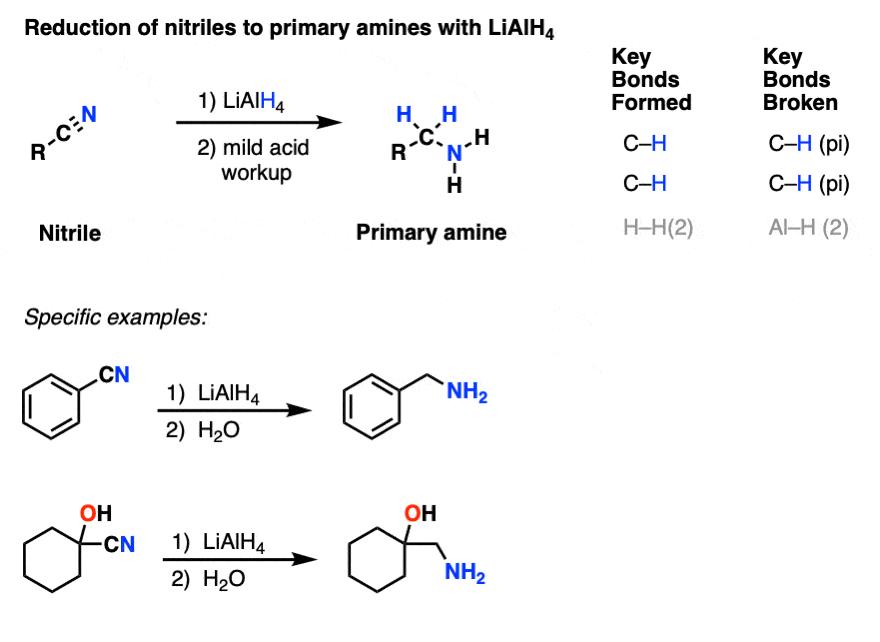
6. Reduction of Nitriles to Primary Amines by LiAlH4
Like the sledgehammer it is, LiAlH4 just keeps smashing through every functional group we’ve thrown in its path. Is there anything it can’t reduce?
Bring on the next opponent!
Nitriles require pretty forcing conditions to hydrolyze into carboxylic acids or amides, and their reduction using catalytic hydrogenation requires high temperatures (50-100 °C) and pressures of hydrogen (this ref cites 100 atm of H2).
Can LiAlH4 do it?
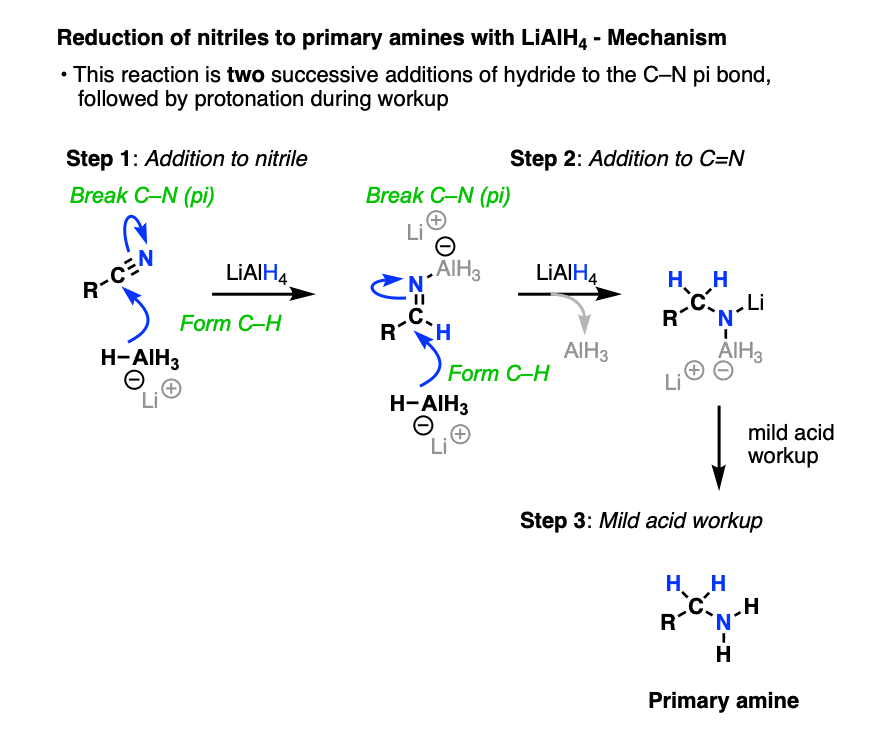
Of course it can! Nitriles are reduced to primary amines.
The first step here is another addition, this time to break a C-N (pi) bond and form an imine species. A second addition of hydride gives a negatively charged nitrogen intermediate that is protonated to a primary amine during workup.
(Note 6 - it’s possible to get the reduction to stop after one addition of hydride by using DIBAL).
You may recall that the cyanide ion CN(-) is an excellent nucleophile that will perform SN2 reactions with alkyl halides. So performing an SN2 followed by LiAlH4 reduction is one way of converting alkyl halides into chain-extended primary amines.
7. Other reactions of LiAlH4
But wait - there’s more!
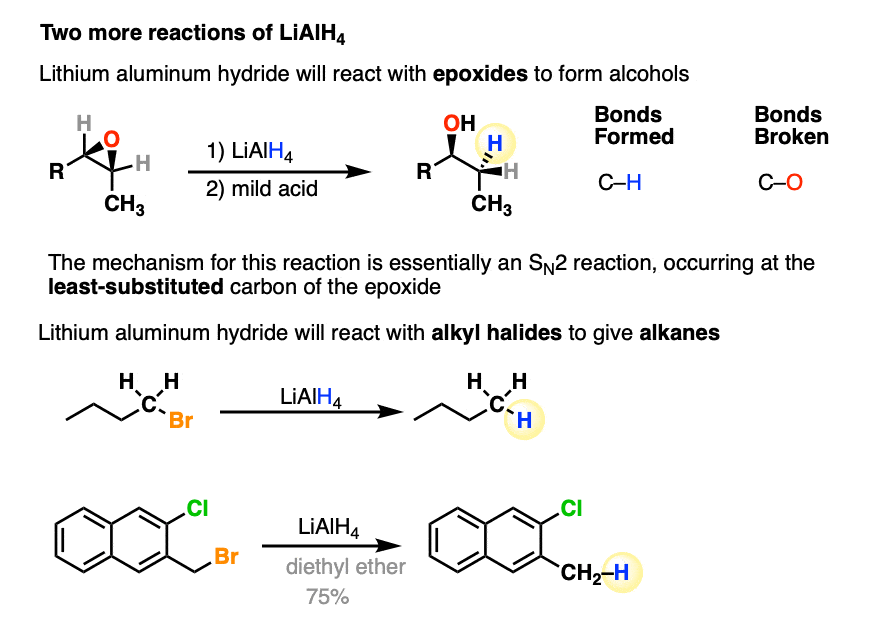
- LiAlH4 will reduce epoxides to alcohols, which you can think of as being like an SN2 reaction. Like other negatively charged nucleophiles, it adds to epoxides at the least substituted carbon. (See article - Opening of Epoxides With Base)
- LiAlH4 will also, in certain cases, reduce alkyl halides to alkanes, in the order I > Br > Cl > F.
It can also reduce azides to primary amines. (See article - Some Reactions of Azides)
One functional group LiAlH4 will not touch is ethers.
8. Summary
- LiAlH4 is the T-rex of reducing agents. If your functional group absolutely, positively, has to be reduced overnight, use LiAlH4. It’s an all-purpose hydride-adding beast that is powerful, readily available, and relatively cheap.
- If you need a reducing agent that will gently tickle a hydride on to your molecule with a feather-light touch, use something else.
- Various gentler derivatives of LiAlH4 such as DIBAL and LiAlH(Ot-Bu)3 have been developed and are much more selective.
- For reduction of aldehydes and ketones, sodium borohydride (NaBH4) is perfectly fine.
Notes
Note 1. LiBH4 is more reactive than NaBH4 due to the presence of the more Lewis-acidic lithium ion. This helps to activate the carbonyl toward nucleophilic attack, much like how protonated carbonyls are more reactive towards nucleophiles than are neutral carbonyls. LiBH4 is not as reactive as LiAlH4, but it will reduce esters to alcohols.
Note 2. Here are some practical reasons for preferring NaBH4 to LiAlH4 .
NaBH4 is generally available as pellets. Left out in the air for a short period, these pellets will absorb water from air and eventually form a little puddle of liquid, but will not spontaneously combust. NaBH4 can be used in cold alcoholic solvents such as EtOH and CH3OH without incident. The workup of sodium borohydride reductions is very simple - usually just quench with saturated ammonium chloride and be done with it.
In contrast, LiAlH4 is a fine grey powder that forms dust clouds upon the slightest disturbance, and one has to hold one’s breath to avoid getting it in the lungs. It reacts violently with water and alcohols and is best used with dry ethereal solvents. Aluminum salts form difficult emulsions, making extraction in a separatory funnel tedious unless the aluminum salts are hydrolyzed with base. The usual procedure (the Fieser workup) goes as follows.
For a reduction using n grams of LiAlH4, one carefully adds dropwise
- n mL of water
- n mL of 15% NaOH solution, and
- 3n mL of water
Upon stirring for 15-30 minutes this should provide a white precipitate that can then be filtered off.
An alternative procedure calls for solid hydrated sodium sulfate (Glauber’s salt, NaSO4•10H2O) to be added portionwise until the salts become white. (Ref).
Note 3. One person I knew (who shall remain nameless) liked to work late at night by himself. One time he weighed out his LiAlH4 for a reduction reaction and left the grey powder on a weighing boat on the laboratory balance. At some point, before adding it to his reaction, he decided to have a little nap at his desk. Not long afterwards he was rudely awakened by the smell of burning plastic from the spontaneous combustion of LiAlH4, which had consumed the ($4000) analytical balance while he slept. Thankfully nobody was injured and the lab was otherwise unscathed.
Note 4. The Al-O bond is very strong (128 kcal/mol), and since aluminum has an empty p-orbital for pi-bonding, the actual leaving group might be something akin to O=AlH2(-) which is considerably less basic than, say, O(2-)Li2
Note 5. The aldehyde can be formed under some conditions. For example using the strong reducing agent super-hydride (Lithium tri sec-butylborohydride) at low temperatures, the aldehyde can be obtained.
A special class of amides known as Weinreb amides will undergo mono-addition by organometallic reagents (including hydride sources). These reactions work because the nitrogen contains a Lewis basic OCH3 group that can coordinate with the metal and help to stabilize the tetrahedral intermediate, preventing elimination.
Note 6. Nitriles will undergo partial reduction with the use of DIBAL-H. They can also undergo addition by Grignard reagents to give ketones after hydrolysis of the resulting imine.
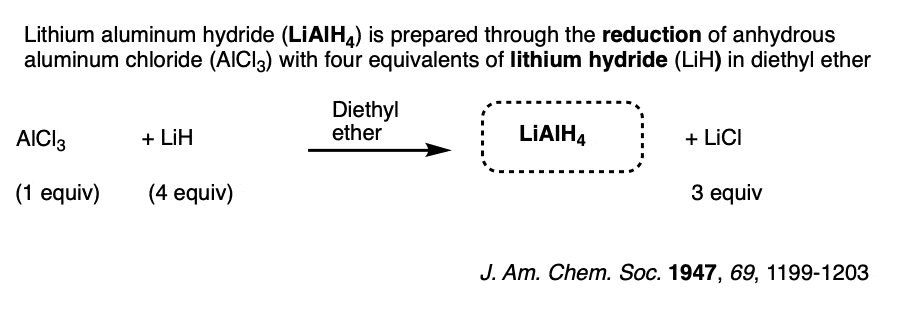
Note 7. LiAlH4 is usually prepared through the reduction of a solution of AlCl3 in ether by lithium hydride (LiH). (Reference).
We generally don’t think of the hydride ion in NaH and LiH as a good nucleophile, but given a strong enough Lewis base, it can act as one.
Quiz Yourself!
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
Become a MOC member to see the clickable quiz with answers on the back.
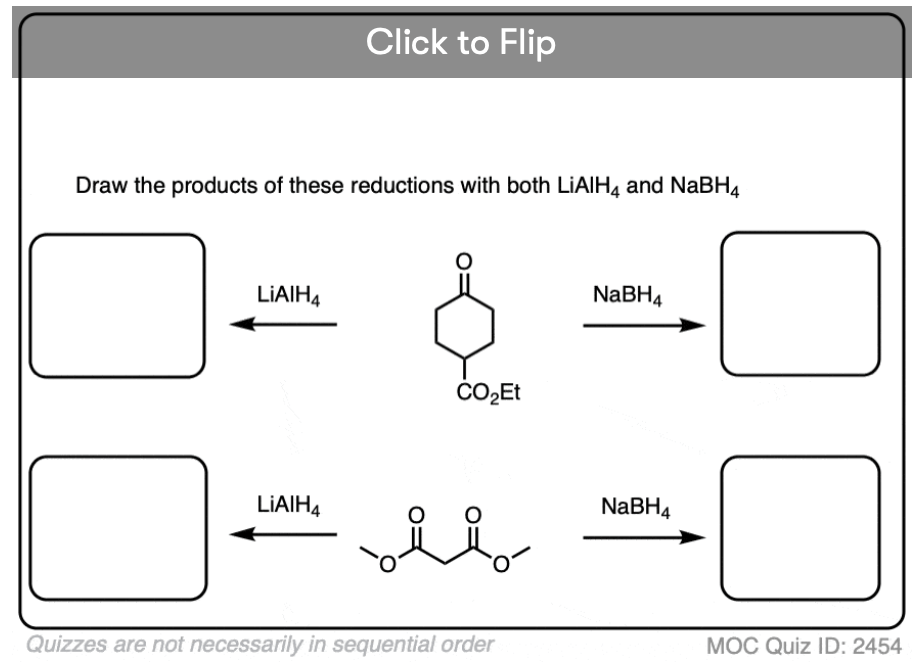
Become a MOC member to see the clickable quiz with answers on the back.
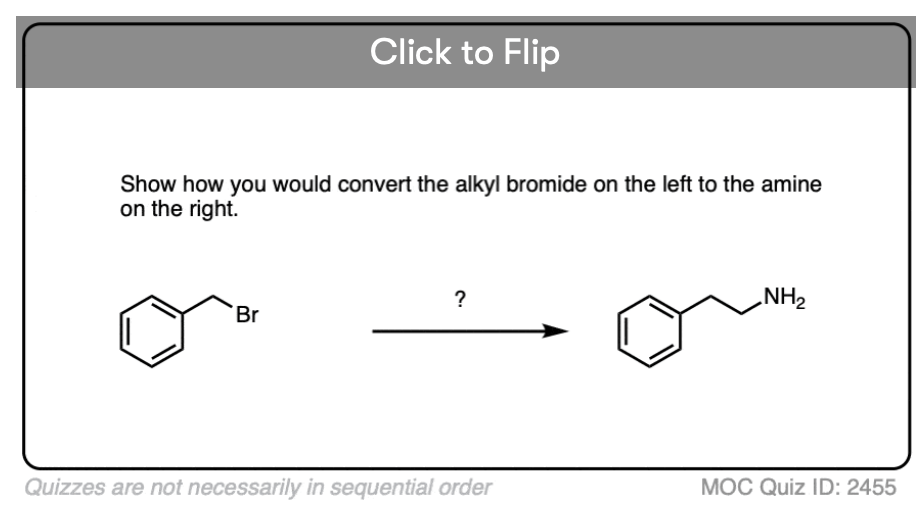
Become a MOC member to see the clickable quiz with answers on the back.
(Advanced) References and Further Reading
For more context on reduction, this handout (PDF) on reducing agents from Prof. Andrew G. Myers’ Chemistry 115 course at Harvard is an extremely useful overview
- Lithium Aluminum Hydride, Aluminum Hydride and Lithium Gallium Hydride, and Some of their Applications in Organic and Inorganic Chemistry A. E. Finholt, A. C. Bond Jr., and H. I. Schlesinger Journal of the American Chemical Society 1947 69 (5), 1199-1203 DOI: 10.1021/ja01197a061 First report on preparing LiAlH4, from AlCl3 and LiH.
- Reduction of Organic Compounds by Lithium Aluminum Hydride. I. Aldehydes, Ketones, Esters, Acid Chlorides and Acid Anhydrides Robert F. Nystrom and Weldon G. Brown Journal of the American Chemical Society 1947, 69 (5), 1197-1199 DOI: 10.1021/ja01197a060 An early paper on LiAlH4 that covers its reaction with functional groups containing C=O double bonds.
- Lessons Learned─Lithium Aluminum Hydride Fires Craig A. Merlic, Carl J. Ferber, and Imke Schröder ACS Chemical Health & Safety 2022 29 (4), 362-365 DOI: 10.1021/acs.chas.2c00035 Lithium aluminum hydride pellets should never be ground in a mortar and pestle! This article describes several fires that occurred as a result of this treatment.
- 2-(1-PYRROLIDYL)PROPANOL Robert Bruce MoffettOrg. Synth. 1953, 33, 82 DOI: 10.15227/orgsyn.033.0082
- Total synthesis of (+)-calyculin A David A. Evans, James R. Gage, and James L. Leighton Journal of the American Chemical Society 1992, 114 (24), 9434-9453 DOI: 10.1021/ja00050a024 The reduction of 55 in this total synthesis is the selective reduction of a methyl ester to an alcohol in the presence of an amide using LiAlH4.
- Daphniphyllum alkaloids. 15. Total syntheses of (.+-.)-methyl homodaphniphyllate and (.+-.)-daphnilactone A Clayton H. Heathcock, Roger B. Ruggeri, and Kim F. McClure The Journal of Organic Chemistry 1992, 57 (9), 2585-2594 DOI: 10.1021/jo00035a012 LiAlH4 can also be used to reduce lactones, removing the cyclic structure. In this paper, the reduction of lactone 18 is effected using LiAlH4, yielding two alcohols.
- 1,2-Benzenedimethanol Ryo Oi and K. Barry Sharpless Org. Synth. 1996, 73, 1 DOI: 10.15227/orgsyn.073.0001 The first step in this procedure is the reduction of diethyl phthalate to 1,2-benzenedimethanol with LiAlH4 in 93% yield.
- N,N-Dimethylcyclohexylmethylamine Arthur C. Cope and Engelbert Ciganek Org. Synth. 1959, 39, 19. DOI: 10.15227/orgsyn.039.0019 This procedure shows the reduction of a tertiary amide to a tertiary amine with LiAlH4 in refluxing ether (15h).
Link nội dung: https://ohanapreschool.edu.vn/alcl3-nh3-h2o-pt-ion-a19298.html